
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.
German researchers have already made a breakthrough in the development of cost-effective and high-efficiency solar fuel equipment, which can store nearly 5% of solar energy in the form of hydrogen.
Artificial photosynthesis, the process of separating water into hydrogen and oxygen using light, can make solar energy stored as hydrogen. Hydrogen can be used directly as fuel, either in the form of methane, or with a fuel cell.
The Helmholtz Berlin Center (HZB) collaborated with the scientists of TU Delft in the Netherlands to develop this device using simple photovoltaic cells and photoanodes made of metal oxide vanadium bismuth (or BiVO4), successfully stored nearly 5% of solar energy as hydrogen.
A small amount of tungsten atoms were added to the photoanode BiVO4, sprayed onto a piece of conductive glass, and coated with an inexpensive cobalt organophosphate.
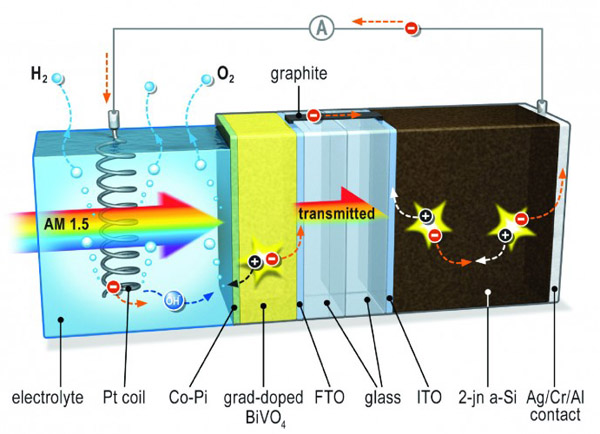
Roel van de Krol, head of the Helmholtz Center for Solar Energy Research at the Berlin Center, said that basically we achieved the best of both worlds. We first used a chemically stable and low-cost metal oxide plus a silicon-based thin film solar cell, and then we created a cost-effective, highly stable, and highly efficient solar fuel device.
The potential for such research is enormous. In Germany, for example, the researchers said that solar power performance is 600 watts per square meter, so theoretically speaking, such an hour of light, this 100 square meter system can store 3kWh of electricity in the form of hydrogen. This power can then be used at night or when needed.
More minimalistic design
The R&D team used a relatively simple silicon-based thin film solar cell and added a metal oxide layer. The oxide layer is only part of the cell sheet in contact with water and forms oxygen as a photoanode. At the same time, this helps prevent the corrosion of silicon-based batteries. Researchers systematically examined and optimized processes such as light absorption, charge separation, and water molecule decomposition. According to van de Krol, when the photoanode is made of yttrium vanadate, the efficiency of converting solar energy to chemical energy can reach 9%. Using cobalt-organic phosphates, the R&D team has significantly accelerated the oxygen formation process.
the biggest challenge
However, according to the research team, the biggest challenge is to separate the charge from the vanadium vanadate. Even though metal oxides are inexpensive and stable in nature, the charge carriers can quickly recombine so that water cracking does not occur. Van de Krol and his team managed to solve this problem by adding tungsten atoms to the yttrium vanadate film in a very special way.
This creates an internal electric field that helps prevent recombination of the charge carriers. By repeatedly spraying different tungsten onto the glass, a highly efficient photo-activated metal oxide film about 300 nm thick was created.
Van de Krol said that we do not quite understand why yttrium vanadate works better than other metal oxides. However, we have found that more than 80% of incident photons can generate currents that can generate unexpectedly high currents and create a new record for metal oxides.
The next challenge is to expand these systems to several square meters so that a corresponding amount of hydrogen can be produced. (Compiled by Tao Shuhua)
April 20, 2024
April 20, 2024
April 20, 2024
April 20, 2024
Gửi email cho nhà cung cấp này
April 20, 2024
April 20, 2024
April 20, 2024
April 20, 2024

Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.

Fill in more information so that we can get in touch with you faster
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.